ECG/EKG Basics: Understanding the Heart’s electrical conductive system through Action Potentials:
Learn about the basics of the heart's electrical conduction system and the basics of how the heart generates ECG waveforms through action potentials.
ECG/EKG
Avya Patel
4/27/20255 min read
ECG/EKG Basics: Understanding the Heart’s electrical conductive system through Action Potentials:
Introduction to Cardiac Conduction
Importance of the electrical system in heart function
The heart’s electrical system is crucial for coordinating its pumping action across all of the heart’s muscles- the myocardium. It works by generating and conducting electrical signals that stimulate the heart muscles to contract and relax in a coordinated manner. This system controls both the heart rate and the rhythm of the heartbeat. These electrical systems arise from a series of cardiac action potentials, originated by the SA (sinoatrial node).
Brief overview of the conduction pathway (SA node → Purkinje fibers)
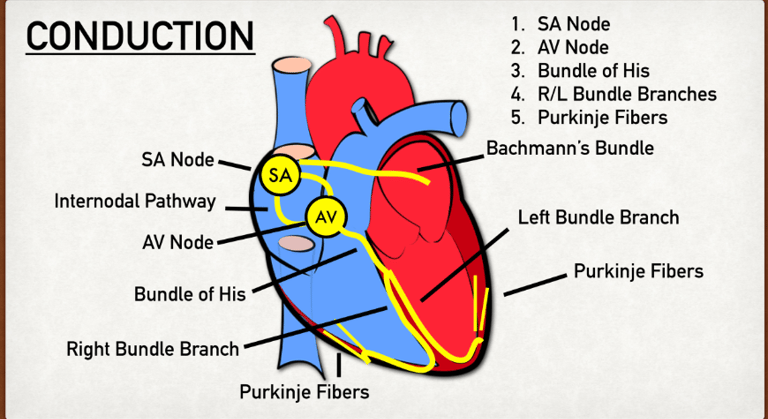
The diagram above displays the conduction pathways from 1-5.
The SA (Sinoatrial) node is a group of pacemaker cells that are located in the upper wall of the right atrium near the superior vena cava. The SA node is the primary node/ origin of a conduction pathway.
Generates 60-100 impulses per minute, setting the pace of the heartbeat, hence the name “pacemaker.”
Atrial Conduction Pathways sends the impulse generated by the SA node to the walls of the left atrium to ensure the contraction of both right and left atrium at the same time.
The AV (Atrioventricular) node is located between the atria and ventricles.
Can take over the generation of electrical impulses if the SA node fails to do so at a slower rate of 40-60 impulses per minute.
It slows the electrical signal briefly (~0.1 second) to allow the ventricles time to fill completely before they contract, without this, the blood flow would be inefficient.
The Bundle of His is split into two pathways, the right and left bundle branches and runs through the interventricular septum to the apex of the heart.
The right and left bundle branches direct the impulse from the AV node to the respective, right and left ventricles.
The Purkinje Fibers are the final part in the conduction pathway. They are a network of fibers that spread through the ventricles which conduct the electrical impulse very rapidly to all parts of the ventricular myocardium, ensuring a strong, coordinated contraction of the ventricles.
Cardiac Action Potentials
Definition of action potential
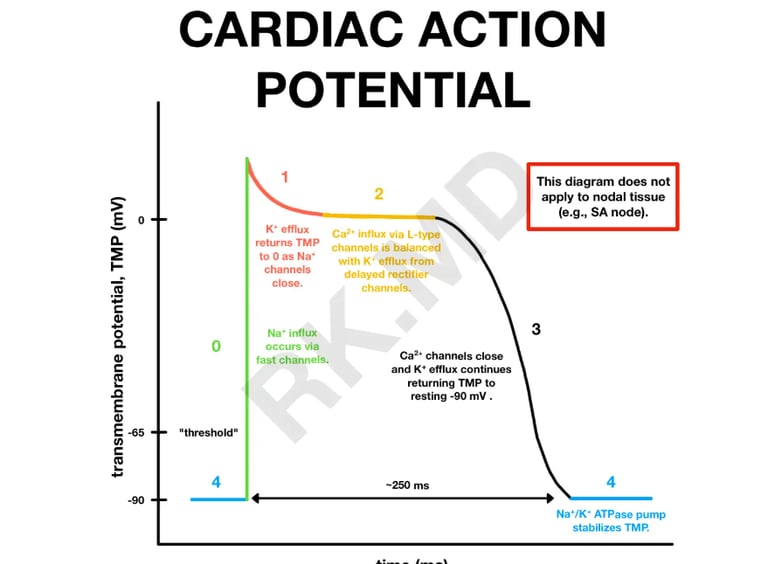
The cardiac action potential is a brief voltage change across the membrane of the heart through cardiac myocytes. This triggers sudden cardiac contraction a result of the movement of charged ions, like sodium, potassium, and calcium, across the cell membrane through specific protein channels.
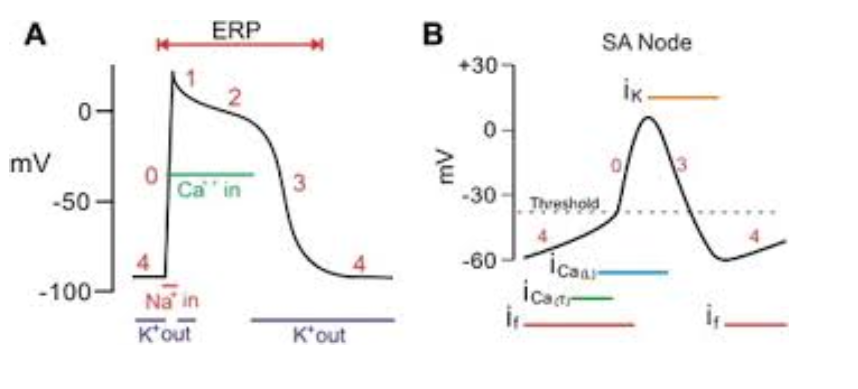
The action potential of cardiac myocytes is measured in millivolts (mV)- measured by the mV of the intracellular fluid. On average, the cardiac action potential of cardiac myocytes starts at -90mV (‘-’ mV is due to the high concentration of negatively charged ions inside the cell).
Since the SA node sets the pace for muscle contractions, its cardiac action potential starts at -60 mV in order to rapidly develop action potentials, hence the different shape of action potentials of the SA node below.
The act of depolarization means ‘increasing the charge of the cell membrane→ more +’, normally, the action potential depolarizes to around +30mV (the intracellular fluid is more positively charged than negatively). Repolarization means ‘decreasing the charge of the cell membrane→ more -’, repolarization of cardiac myocytes occurs until -90mV (its resting membrane potential), whereas the SA node repolarizes to -60mV (its resting membrane potential).
(Rishi. (2021, January 25). Cardiac Action Potential. RK.MD. https://rk.md/2021/cardiac-action-potential/)
Phases of the Action Potential
Phase 0: Rapid depolarization (Na⁺ influx)
Trigger:
The action potential is initiated when the membrane potential reaches a threshold, often by a signal coming from adjacent cells (like a Purkinje fiber).
Main Event:
Fast voltage-gated sodium (Na⁺) channels open very quickly.
What Happens:
Massive influx of Na⁺ ions into the cell.
The membrane potential rapidly shoots up from about -90 mV to around +20 to +30 mV.
Key point:
This is the sharp upstroke you see on action potential graphs.
The rapid Na⁺ entry depolarizes the inside of the cell (makes it more positive).
Phase 1: Initial repolarization
Trigger:
Right after Phase 0 finishes.
Main Events:
Inactivation of sodium channels (they quickly shut after opening).
Transient outward potassium (K⁺) channels open briefly.
What Happens:
A small amount of K⁺ ions exits the cell.
This causes a slight dip in membrane potential — not full repolarization, just a little notch.
Key point:
This phase is brief and sets the stage for the next important phase (plateau).
Phase 2: Plateau (Ca²⁺ influx, K⁺ efflux)
Trigger:
After initial repolarization.
Main Events:
L-type calcium channels open → Ca²⁺ enters the cell.
Potassium channels stay open → K⁺ exits the cell.
What Happens:
Calcium influx (Ca²⁺ in) tries to depolarize (make inside more positive).
Potassium efflux (K⁺ out) tries to repolarize (make inside more negative).
These two flows balance each other, so the membrane potential stays relatively flat.
Key point:
This plateau is essential because it prolongs contraction, allowing enough time for blood ejection from the ventricles.
(Also, this long phase prevents premature contractions — giving the heart enough time to refill.)
Phase 3: Repolarization (K⁺ efflux)
Trigger:
After the plateau phase.
Main Event:
Calcium channels close (so no more Ca²⁺ influx).
Potassium efflux dominates.
What Happens:
K⁺ keeps flowing out through open potassium channels.
Without incoming Ca²⁺ to balance it, the cell becomes more negative again.
Membrane potential returns toward resting levels (~ -90 mV).
Key point:
Full repolarization happens here, resetting the cell for the next action potential.
Phase 4: Resting potential
Trigger:
After repolarization is complete.Main Event:
Potassium permeability dominates.
Na⁺/K⁺ ATPase pumps work to maintain ionic gradients (3 Na⁺ out, 2 K⁺ in).
No significant Na⁺ or Ca²⁺ influx.
What Happens:
The inside of the cell remains stable at about -90 mV.
The cell is ready for the next signal.
Key point:
This stable resting phase is why contractile cells don't fire spontaneously (unlike pacemaker cells).
Pacemaker vs. Contractile cells Action Potentials
(B) Pacemaker cells (SA/AV nodes) – automaticity, phases 4, 0, 3
Pacemaker cells (SA and AV nodes) are specialized for automaticity — meaning they can spontaneously generate action potentials without any external stimulation.
They have three phases of action potentials: Phase 4, Phase 0, and Phase 3.
Phases of Pacemaker Cells:
Phase 4 (Spontaneous Depolarization):
Key Feature: Slow, spontaneous depolarization toward threshold.
Cause:
Funny currents (If) — carried by sodium (Na⁺) ions — leak into the cell.
Also some calcium (Ca²⁺) influx via T-type calcium channels.
Automaticity happens because the membrane potential never stays stable — it slowly creeps up toward threshold.
Phase 0 (Depolarization):
Key Feature: Rapid depolarization.
Cause:
Opening of L-type calcium channels (not sodium like in contractile cells!).
Calcium (Ca²⁺) rushes into the cell, causing a sharp upstroke in membrane voltage.
Phase 3 (Repolarization):
Key Feature: Return to resting potential.
Cause:
Opening of potassium (K⁺) channels, allowing K⁺ to flow out.
Membrane potential becomes negative again.
(A) Contractile cells (atria/ventricles) – phases 0 to 4
Contractile cells (atria and ventricles) are designed to generate powerful contractions, not automaticity.
They need an outside stimulus (usually from the Purkinje fibers) to fire an action potential.
They have five phases: 0, 1, 2, 3, and 4.
Phases of Contractile Cells:
Phase 4 (Resting Membrane Potential):
Key Feature: Stable resting membrane potential (~ -90 mV).
Cause:
High potassium (K⁺) permeability.
No significant sodium or calcium inflow.
Phase 0 (Rapid Depolarization):
Key Feature: Sharp spike upward.
Cause:
Fast sodium channels open.
Massive influx of sodium (Na⁺) ions.
Phase 1 (Initial Repolarization):
Key Feature: Small dip after the peak.
Cause:
Inactivation of sodium channels.
Brief, outward flow of potassium (K⁺).
Phase 2 (Plateau Phase):
Key Feature: Membrane potential stays relatively flat.
Cause:
Balance between calcium (Ca²⁺) influx via L-type calcium channels and potassium (K⁺) efflux.
This plateau maintains contraction and allows for good ejection of blood.
Phase 3 (Repolarization):
Key Feature: Membrane potential drops back down.
Cause:
Calcium channels close.
Potassium (K⁺) efflux dominates, returning the cell to resting potential.